Which of the Following Describes the Bohr Atomic Model
The Bohr Model considers electrons to have both a known radius and orbit which is impossible according to Heisenberg. The most appropriate statement that describes the Neils Bohrs atomic model is.

Atomic Models Scientist And Years Solid Sphere Model Plum Pudding Model Planetary Model Quantum Model Ch Planetary Model Chemistry Education Plum Pudding Model
Such orbits are called stationary orbits.

. Use Bohrs model to draw a sodium Na atom and a chlorine Cl atom. Which of the following statements is not true of the atomic model of bohr. B An atom with 9 protons in the nucleus with 8 electrons on the first shell and 11 electrons on the second shell and 9.
Atoms shrink Electrons are excited by the light from a lower energy orbit to a higher one Electrons relax from a higher energy orbit to a lower one Protons are excited by the light from a lower energy orbit to a higher one O Protons and. Up to 24 cash back Atomic Model Worksheet. Bohrs model consists of a small nucleus positively charged surrounded by negative electrons moving around the nucleus in orbits.
In an atom the electrons revolve around the nucleus in certain definite circular paths called orbits or shells. Various postulates of Bohrs atomic model are. Which of the following is NOT a developer of an atomic model.
Neils Bohr put forward the following postulates about the model of an atom. Unlike earlier models the Bohr Model explains the Rydberg formula for the spectral emission lines of atomic hydrogen. I Only certain special orbits known as discrete orbits of electrons are allowed inside the atom.
Include in your explanation ion and ionic bond formation. He developed the model after studying the way glowing hot hydrogen gives off light. The electrons revolve in definite orbits that are present around the nucleus.
Ii While revolving in discrete orbits the electrons do not radiate energy. Bohrs model had more electrons around the nucleus. Hot springs or volcanicactivityBased on the picture above arrange in proper order the following steps to be followed on how togenerate electricity in a geothermal power plants.
Every circular orbit will have a certain amount of fixed energy and these circular orbits were termed orbital shells. It was while Bohr was working in England in 1913 that he developed this atomic model. In 1913 Bohr suggested that electrons could only have certain classical motions.
Florianmanteyw and 16 more users found this answer helpful. - 2396813 Mpyc12345 Mpyc12345 22092019 Science. Bohrs Model of an Atom.
Using your model explain what happens when sodium reacts with chlorine to form table salt. The Bohr Model contains some errors but it is important because it describes most of the accepted features of atomic theory without all of the high-level math of the modern version. What describes the bohr atomic model.
Due to this high probability orbits are replaced by the term orbital. The nucleus contains protons and neutrons and is orbited by electrons. These orbits were represented either as 1 2 3 or K L M Nand so on.
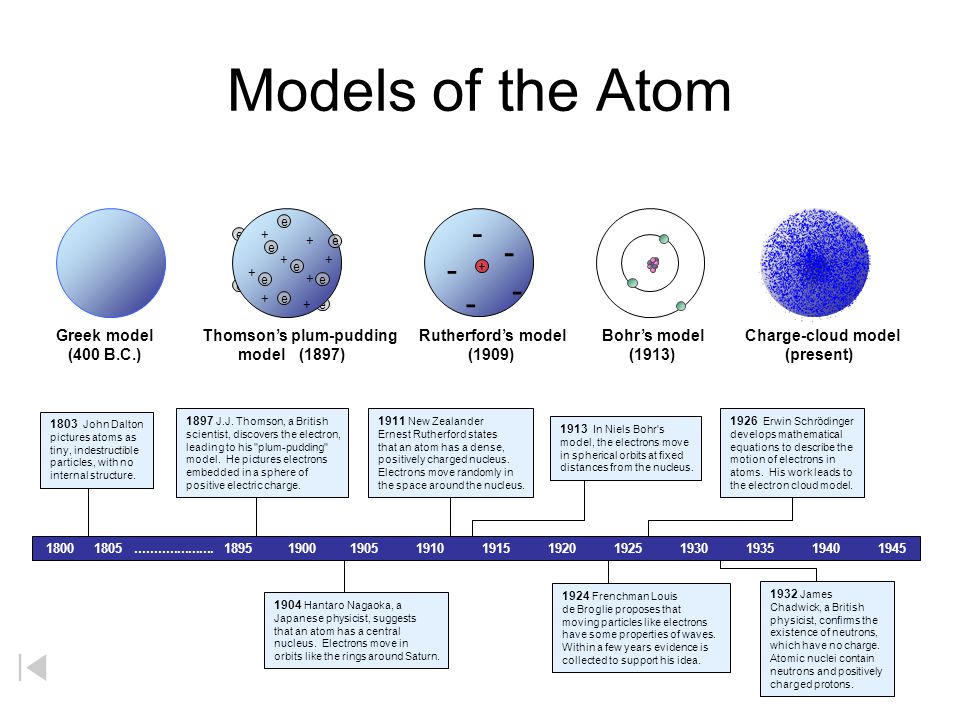
The Bohrs postulates - 1 An electron can revolve around the nucleus in certain fixed orbits of definite energy without emission of any radiant energy. Bohrs model shows electrons at certain distances from the nucleus. Electrons in atoms orbit the nucleus.
According to the Bohrs atomic model the electrons revolve around the nucleus in a fixed orbit. According to the new current Quantum atomic model An orbital is a region of space in an atom where the. The Bohr Model of the atom was a slightly more intuitive model that human language used to describe however it was also inaccurate.
Electrons revolve around the nucleus in a fixed circular path termed orbits or shells or energy level. Which statement correctly describes the quantum number n with reference to the Bohr model of the hydrogen atom. Which of the following statements best describes how the Bohr model of an atom is different from this one.
It is connected with a fixed value of momentum. Select all that apply. When an incandescent light bulb is lit it gives off all the different wavelengths of light.
Postulates of Bohrs atomic model. The Bohr model of the atom electrons travel in defined circular orbits around the nucleus. An atom consists of a central nucleus with proton neutrons and electrons orbiting in levels of high probability.
2 An electron can make a transition from a stationary state of higher energy E_2 to a state of lower energy E_1 and in doing so it emits a single photon of frequency nu E_2 -. The nucleus contains protons and neutrons and is orbited by electrons. See answers 2 Best Answer.
The orbits are termed as stationary orbit. Which of the following statements is true about Bohrs planetary model of the atom. Which of the following statements correctly describes the fluorine atom using the RutherfordBohr model.
Which of the following statements most accurately describes atomic absorption of light using the Bohr model of the atom. Bohr found that an electron located away from the nucleus has more energy and electrons close to the nucleus have less energy. Each shell or orbit corresponds to a definite energy.
Put A to E on the blank provided in each. Bohr assumes that an electron in an atom is positioned at a specific detachment from the nucleus and is revolving round it with explicit velocity ie. The orbits are labeled by an integer the quantum number n.
Electrons can jump from one orbit to another by emitting or absorbing energy. A An atom with 9 protons in the nucleus with 2 electrons on the first shell and 7 on the second shell. In the year 1913 Niels Bohr proposed an atomic structure model describing an atom as a small positively charged nucleus surrounded by electrons that travel in circular orbits around the positively charged nucleus like planets around the sun in our solar system with attraction provided by electrostatic forces popularly known as Bohrs atomic model.
The Bohr Atom Model. Therefore these circular orbits are also known as energy levels or energy shells. N indicates the energy level of an electron in the atom.
N can have any positive whole-number value greater than or equal to 1. N is a measure of the difference in energy between two energy levels. The subatomic particles in the Bohr model.
The postulates given by Neils Bohr are. Bohr Atomic Model.

Illustration Of Chemistry Atomic Models Scientific Theory Of The Nature Of Matter Ad Spon Atomic Models Illus Atom Model Atom Drawing Scientific Poster

When The Atom Went Quantum Teaching Chemistry Chemistry Lessons Atom
Comments
Post a Comment